Products
DRUG-DELIVERY PLATFORMS
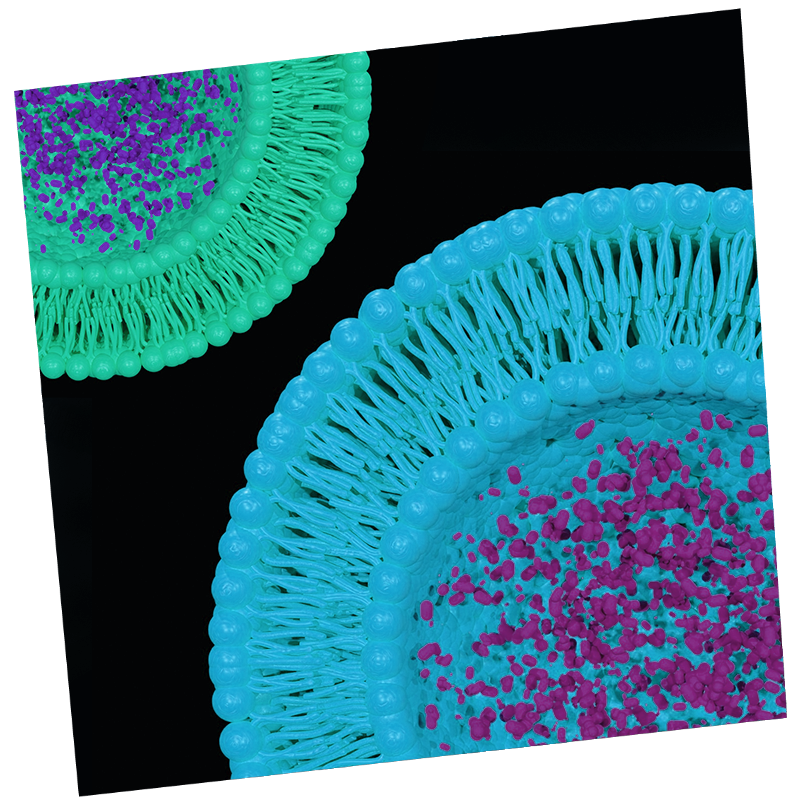
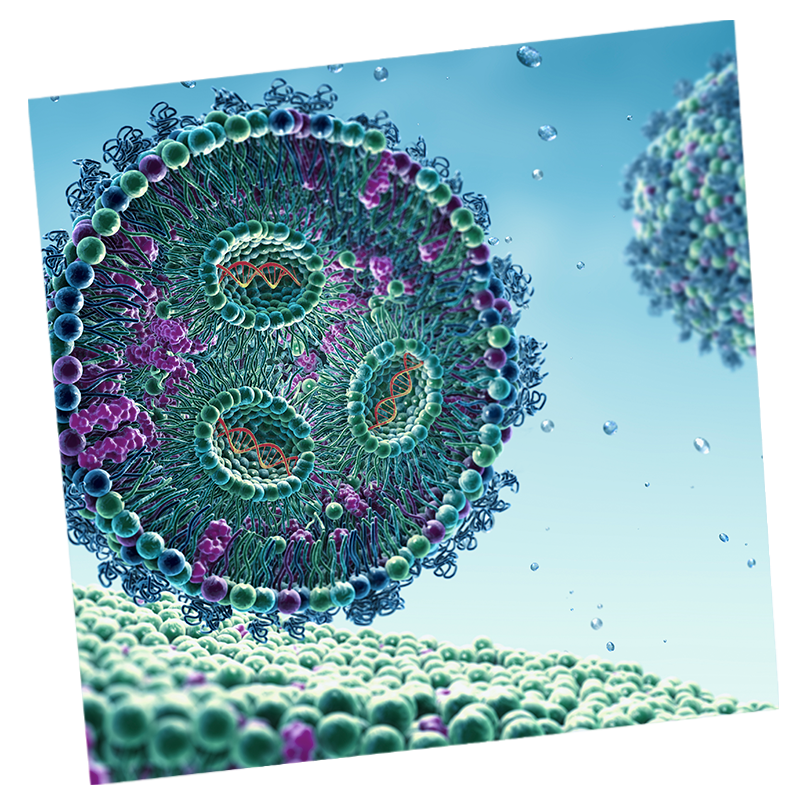
Liposomal encapsulation
Probudur may extend local anesthetic at the wound/incision site, effectively managing post-operative pain for 96 hours as evidenced by non-clinical study data.
We’re using liposomal encapsulation to develop a local anesthetic for:
We’re developing molecules with the MET platform to deliver treatment options for the management of:
Probudur™
Lead Product Candidate
Injectable long-acting liposomal bupivacaine
TARGET MARKET
Postoperative pain
Global market size
(Market Study on Postoperative Pain Management 2022-2031, Persistence Market Research)
DELIVERY PLATFORM
Liposomal encapsulation
POTENTIAL BENEFITS
- Animal studies demonstrated 96 hours of local anesthetic activity at wound site
- May eliminate the need for opioids after surgery
- May reduce associated costs and length of hospital stay
- Produced no evidence of motor or sensory nerve damage at a dose that was 10 times higher than free bupivacaine in animal studies
“FDA takes steps aimed at fostering development of non-addictive alternatives to opioids for acute pain management lasting up to 30 days, in response to some form of tissue injury, such as trauma or surgery.”
U.S. Food and Drug Administration
FEBRUARY 2022

MECHANISM OF ACTION
Bupivacaine works when injected into the wound by binding to the ‘sodium channel’ and blocking the influx of sodium ions; this inhibits nerve conduction and prevents pain signals from reaching the brain after surgery. In our pre-clinical studies, the Liposomal Bupivacaine acts as an ultra-long acting formulation with a 96-hour duration at the wound site.
NEW DRUG APPLICATION (NDA)
As a result of our PreIND review, the FDA has indicated that it is reasonable for Virpax to pursue an accelerated 505(b)(2) New Drug Application for Probudur.
USAISR CRADA AWARDED 5/05/22
Virpax entered into a cooperative research and development agreement (CRADA) with the U.S. Army Institute of Surgical Research (USAISR) on May 5, 2022 to evaluate Virpax’s Probudur, an injectable long-acting liposomal bupivacaine in a formulation that is injected at the wound site. In pre-clinical trials, Probudur has shown pain control for 96 hours. The USAISR is the U.S. Department of Defense’s (DOD) primary laboratory for developing solutions for trauma and critical care challenges in combat casualties.
†Engaged Destum Partners to execute a global search for Animal Health partner. See Global Sublicensing Strategy.
TARGET MARKET
Severe Pain, including Post Cancer Pain
Global Market size
(Analgesics Market Research Report by Drug Type Global Forecast 2023-2030, Research and Markets)
DELIVERY PLATFORM
Intranasal Molecular Envelope Technology (MET)
Global Intranasal Market
Intranasal Drug Delivery market to reach
(Analgesics Market Research Report by Drug Type Global Forecast 2023-2030, Research and Markets)
POTENTIAL BENEFITS
- Enkephalins are naturally occurring peptides that target delta receptor agonists in the brain to control severe pain, without the undesirable side-effects associated with mu receptor agonists like morphine. Hence, enkephalins are mu receptor sparing
- Analgesic potential to manage acute and chronic pain, including pain associated with cancer, without the concerns of opioid tolerance, withdrawal, respiratory depression, or euphoria
- Our IND-enabling studies have determined that the MET intranasal delivery formulation bypasses the liver, avoiding potential drug to drug interactions if taking concomitant medicine
MECHANISM OF ACTION
Brain delivery: Molecular Envelope Technology (MET) allows for highly stable nanoparticles to wrap around and encapsulate hydrophobic drugs and peptides, creating a protective molecular “envelope.” MET allows for increased bioavailability of drugs that would otherwise be rapidly degraded. In the case of intranasally administered Envelta™, MET increases dwell time in the nares promoting delivery via the olfactory route across the blood brain barrier and into the central nervous system. Here they target the delta opioid receptors, promoting analgesia (pain relief) without the morphine related side-effects.

NEW DRUG APPLICATION (NDA)
As a result of our PreIND review, the FDA has indicated that it is reasonable for Virpax to pursue a New Chemical Entity (NCE) New Drug Application for Envelta.

NCATS CRADA AWARDED 8/31/20
Virpax advances Envelta development with NCATS under CRADA Agreement to support the development and manufacturing of Envelta. The National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH) has awarded research and development contracts to support Good Manufacturing Practices (GMP) production of drug substance and drug product. as well as to support Good Laboratory Practices (GLP) toxicology, safety studies and preclinical efficacy studies to develop Envelta.
*We may also develop Envelta for a second indication, PES200, which would utilize the same delivery mechanism as Envelta to enable the delivery of a metabolically liable peptide drug (Enkephalin) into the brain for post-traumatic stress disorder. The Envelta IND enabling studies may be cross-referenced to the PES200 IND.
NobrXiol™
Intranasal pharmaceutical-grade cannabidiol (CBD)
Pursuing Grant Opportunities
NobrXiol™
Intranasal pharmaceutical-grade cannabidiol (CBD)
Pursuing Grant Opportunities

TARGET MARKET
Management of Rare Pediatric Epilepsy; Potential Orphan Disease
Global market size
(Global Pediatric Epilepsy Therapeutics Market, 2022-2030, Data Bridge Market Research)

DELIVERY PLATFORM
Intranasal Molecular Envelope Technology (MET)
Global Intranasal Market
Intranasal Drug Delivery market to reach
(Antiepileptic Drugs Market Size, Share, Report | Forecast 2019-2026 Fortune Business Insights)
POTENTIAL BENEFITS
- NobrXiol may utilize significantly less pharmaceutical-grade CBD than current FDA approved oral CBD dosing
- NobrXiol’s nose-to-brain drug delivery platform may provide significantly faster onset of action compared to the current FDA approved oral CBD dosing
- NobrXiol may achieve higher efficiency via the nasal route and reduce peripheral side effects like dose dumping due to high-fat meals
- Since peripheral exposure via the plasma will be reduced by the nose to brain delivery, we believe there will be negligible liver first-pass metabolism
- NobrXiol may not be metabolized in the liver avoiding drug to drug interactions caused by oral pharmaceutical-grade CBD
- By avoiding the first pass metabolism degradation of the CBD, effect Intranasal NobrXiol may eliminate enzymatic degradation or deactivation. Enzymatic deactivation occurs when an enzyme changes the structure of a neurotransmitter so that the receptor no longer recognizes the neurotransmitter

MECHANISM OF ACTION
Highly purified pharmaceutical-grade cannabidiol (CBD), approved in the United States, has demonstrated efficacy with an acceptable safety profile in patients with Lennox-Gastaut or Dravet syndrome. CBD acts on cannabinoid (CB) receptors of the endocannabinoid system, which are found in numerous body areas, including the peripheral system and the central nervous systems, including the brain. The endocannabinoid system regulates many physiological responses of the body and neuronal excitability responses relevant to the pathophysiology of many disease types, including epilepsy.

NEW DRUG APPLICATION (NDA)
Virpax will pursue FDA Fast Track, Orphan Drug Designation and Rare Pediatric Disease Designation as a New Chemical Entity (NCE)
“Over the past 40 years, the Orphan Drug Act (ODA) has established a strong foundation for the biotech community to invest in and develop new medicines for rare diseases”
JANURARY 2023

NINDS Participation Agreement AWARDED 04/19/23
Virpax entered into a participation agreement with the National Institute of Neurological Disorders and Stroke (NINDS), part of the U.S. National Institutes of Health (NIH) Division of Translational Research which conducts and funds research on brain and nervous system disorders. Virpax will be partnering with the Epilepsy Therapy Screening Program (ETSP) whose mission is to identify novel agents to address unmet medical needs in epilepsy, including the identification of next generation products focused on addressing drug resistant epilepsy, disease prevention and modification. Under the participation agreement, NINDS ETSP will evaluate Virpax’s NobrXiol product candidate that is being developed for the management of rare pediatric epilepsy. NobrXiol utilizes a unique intranasal Molecular Envelope Technology (MET) delivery platform for pharmaceutical-grade cannabidiol (CBD).
First Pass Effect
The first pass effect is a phenomenon of drug metabolism whereby the concentration of a drug, specifically when administered orally, is greatly reduced before it reaches the systemic circulation. It is the fraction of drug lost during the process of absorption which is generally related to the liver and gut wall.
“The effects of rare neurodegenerative diseases are devastating, with very few effective therapeutic options available to patients. We recognize the urgent need for new treatments that can both improve and extend the lives of people diagnosed with these diseases.”
ROBERT M. CALIFF, M.D.
FDA Commissioner
About the 505(b)(2) regulatory pathway
The 505(b)(2) regulatory pathway is a type of New Drug Application (NDA) submission that encourages further innovation of drugs that have already received approval from the U.S. Food and Drug Administration (FDA). This pathway allows for faster approval times as it can avoid unnecessary duplication of studies that were already performed on approved drugs.
A 505(b)(2) NDA contains full safety and effectiveness reports, however the FDA can rely on other data not developed by the NDA applicant when considering drug approval. This data, like safety and efficacy information, can come from other studies of previously approved pharmacological agents that are similar in nature.
About a New Chemical Entity (NCE)
A New Chemical Entity (NCE) is a drug that does not contain an active ingredient that has been approved by the U.S. Food and Drug Administration (FDA) with any other application submitted under 505(b)(1) regulatory pathways. NCEs are generally developed during the early discovery stage of the product cycle, then undergo various clinical trials that can transform the active ingredient into a product. All investigations supporting safety and effectiveness, both clinical and non-clinical, under 505(b)(1), are conducted by or on behalf of the Sponsor. The FDA can provide exclusivity to an NCE so no other manufacturer can apply for a product with the same active ingredient for a determined amount of time.
Rare Pediatric Disease (RPD) Designation and Voucher Programs
Under Section 529 to the Federal Food, Drug, and Cosmetic Act (FD&C Act), FDA will award priority review vouchers to sponsors of rare pediatric disease product applications that meet certain criteria. Under this program, a sponsor who receives an approval for a drug or biologic for a “rare pediatric disease” may qualify for a voucher that can be redeemed to receive a priority review of a subsequent marketing application for a different product.
Orphan Drugs
The FDA has authority to grant orphan drug designation to a drug or biological product to prevent, diagnose or treat a rare disease or condition. Orphan drug designation qualifies sponsors for incentives including:
- Tax credits for qualified clinical trials
- Exemption from user fees
- Potential seven years of market exclusivity after approval
Orphan drug designation is a separate process from seeking approval or licensing. Drugs for rare diseases go through the same rigorous scientific review process as any other drug for approval or licensing.