Behind the science
THE DATA BEHIND OUR NON-ADDICTIVE PRODUCT CANDIDATES
Various posters have been presented on Virpax’s scientific advancements and discoveries.
A poster presented at the 2019 Military Health System Research Symposium (MHSRS) on August 21, 2019: Sustained Non-Opioid Pain Management in Prolonged Field Care and Hospital Settings Using Probudur describes how the company’s proprietary liposomal bupivacaine gel demonstrated the ability to provide post-operative pain control for up to 96 hours.
Another poster entitled “Enkephalin as a Potential Analgesic and CNS Modulator“ appeared at PAINWeek 2021. It shows that nanoparticle encapsulation of enkephalin would lead to effective brain delivery and analgesia.
Probudur™
Ultra-Long-Acting Local Anesthetics: Liposomal Encapsulated Bupivacaine
Sustained Non-Opioid Pain Management in Prolonged Field Care and Hospital Settings
PROBUDUR CRYOGENIC TRANSMISSION ELECTRON MICROSCOPY (CRYO-TEM)
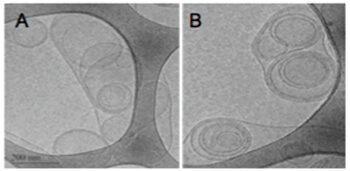
Large Bupisome (LMVV)
Studied in rabbits, rats, dogs, pigs – as well as human pharmacokinetic (PK) data
LMVVs are large structures which have a high level of encapsulated drug (trapped volume 21x higher than SUVs). Part of the bupivacaine is incorporated in the liposomal membrane which releases the drug slowly over time producing long-lasting analgesia.
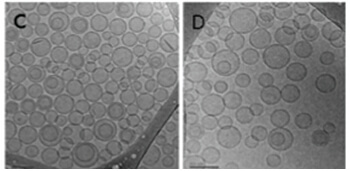
Small Unilamellar Vesicles loaded with Bupivacaine (LMVV)
EFFICACY/SAFETY DATA OBSERVATIONS2
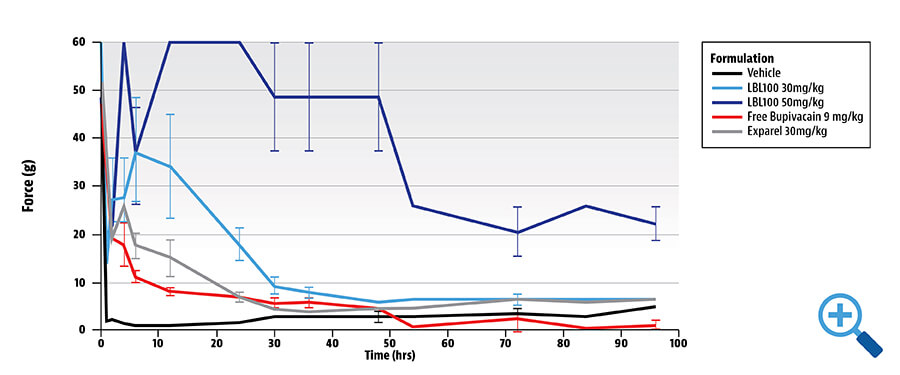
- Pig postoperative pain – superior to bupivacaine and Exparel®, an injectable liposomal bupivacaine
- No effects on wound healing
- Rat nerve block – comparable efficacy to Exparel®
- No morbidity, mortality or toxicity signs, no gross pathological abnormalities; MTD > 100 mg/kg of Probudur bupivacaine (much greater than plain bupivacaine)
- Rabbit: Probudur at a dose of 1.6 mg/kg was demonstrated to be as non-toxic to nerves as saline
- Dog: No morbidity or mortality; EKG within normal limits up to 60 mg/kg; PK revealed low plasma levels of both Probudur and Exparel® with a similar Cmax
DOSE RESPONSE IN HEALTHY VOLUNTEERS
Duration of skin analgesia as assessed by pinprick after subcutaneous injection of 0.5% standard bupivacaine (BUP) or 0.5, 1.0, or 2.0% large multivesicular vesicle (LMVV) liposomal bupivacaine1
PIG: WOUND MODEL: PROBUDUR SUPERIOR TO BUPIVACAINE AND EXPAREL® AT 96 HOURS2
Probudur peak achieved around 6 hours with persistent analgesia noted at 96 hours- 24 hours longer than competitor claims
OPIOID SPARING BENEFITS
ENHANCED RECOVERY AFTER SUGERY (ERAS)
- Rapidly becoming standard of care in perioperative medicine
- Earlier recovery of bowels, bladder, pulmonary system, etc.
REDUCED OPIOID RELATED ADVERSE EVENTS (ORAEs)
- Reduced length of stay
- Less cognitive dysfunction by opioid sparing
- Less nausea, vomiting
HEALTH ECONOMIC OUTCOME BENEFITS
- Cost savings
- Earlier ambulation
- Earlier discharge
- Improved medical outcomes
- Improved patient satisfaction
Conclusion
- Effective analgesia enhances recovery and reduces postoperative morbidity
- Depot formulations of local anesthetics remain at the site of injection and release drug slowly over time
- Results in prolonged analgesic effect with a reduction in plasma concentrations compared to standard anesthetics
- Probudur demonstrated 96-hour activity, efficacy, tolerability and safety in multiple animal models
- All ingredients are biocompatible, biodegradable and GRAS
- A successful pre-IND meeting with FDA allows for a 505(b)(2) FDA regulatory approval pathway
- Probudur is a promising ultra-long-acting depot local anesthetic for intraoperative use with the potential to spare opioids and minimize opioid related adverse effects
ENVELTA™
Enkephalin as a Potential Analgesic and CNS Modulator
INTRODUCTION
- Endogenous peptides with analgesic potential include endorphins via binding to the mu receptor, dynorphins via the kappa receptor and enkephalins via the delta receptor
- Kappa and delta opioid agonists have been shown to provide analgesic benefit while sparing mu agonist toxicity1
- Preclinical data supports analgesia from enkephalin without significant opioid tolerance or drug liking
- Typically, exogenous enkephalins are rapidly degraded and have difficulty accessing the CNS2
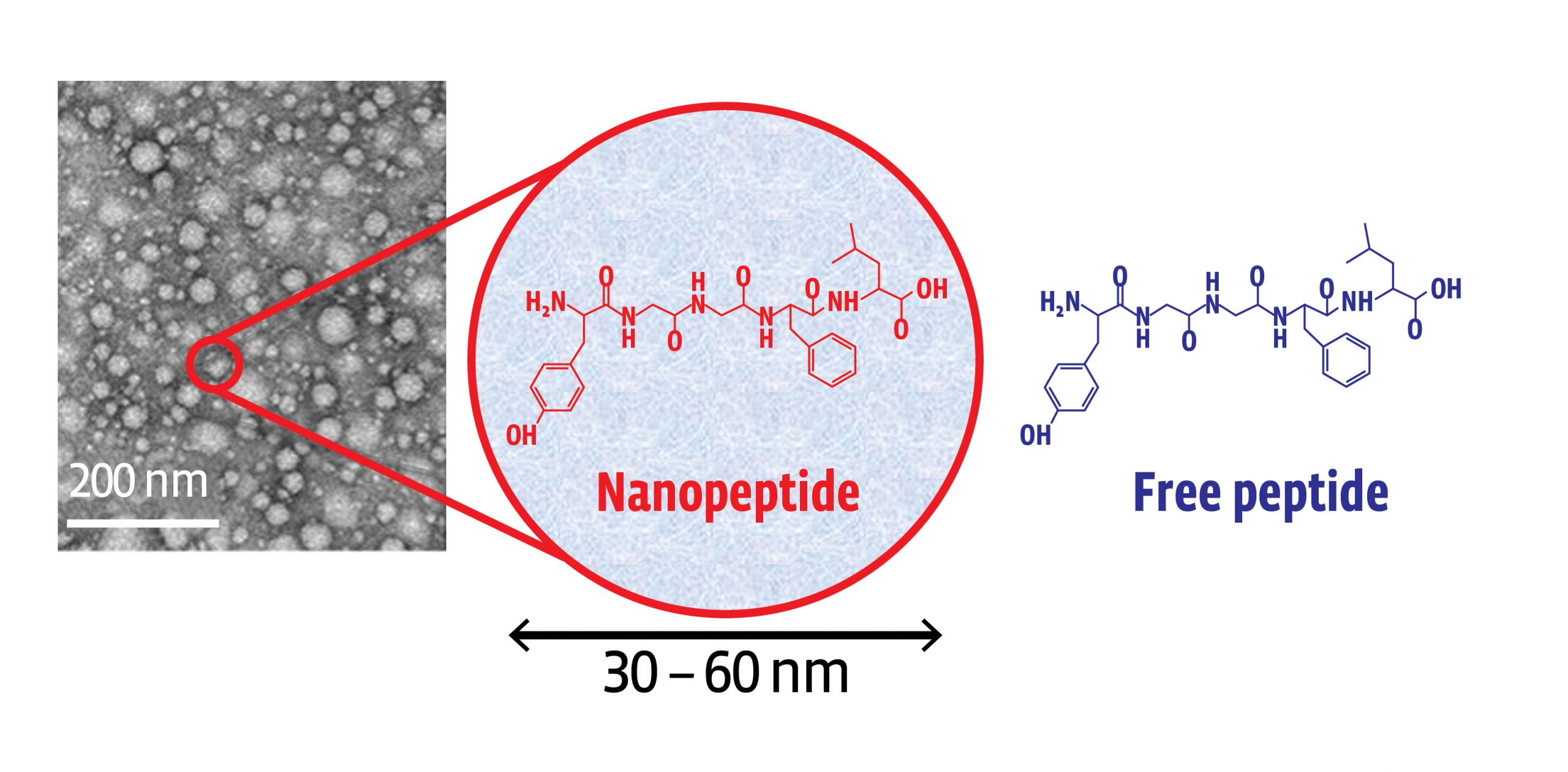
- Using a novel encapsulation method known as Molecular Envelope Technology (MET), leucine-enkephalin, or L-ENK can be delivered via an intranasal formulation of “protected” nanoparticles that can avoid first pass metabolism issues by being delivered directly to the brain via the nose
MET increases dwell time in the nares promoting delivery via the olfactory route across the blood brain barrier and into the central nervous system
Preclinical data
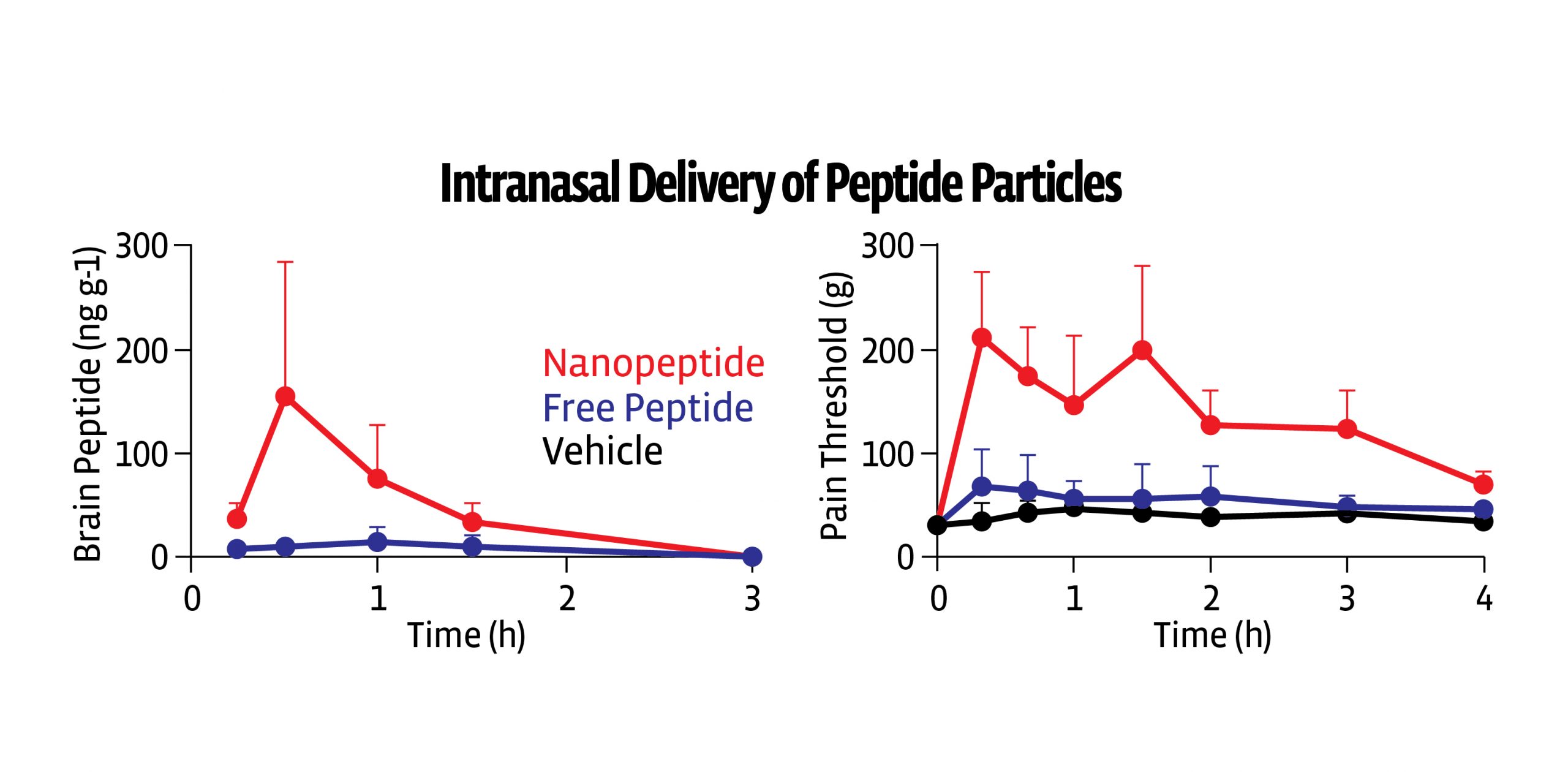
- When studied in rats, polymer nanoparticles were able to transport MET encapsulated L-ENK to the brain via the intranasal route
- Animals dosed with encapsulated L-ENK nanoparticles showed a strong anti-nociceptive response in multiple assays of evoked pain, including hot plate, Freund’s adjuvant (CFA), and spinal nerve ligation
- No analgesic tolerance was noted
- Animals dosed intranasally with unencapsulated LENK did not show an analgesic response. L-ENK was effective in morphine tolerant animals
- Conditioned place preference, a preclinical behavioral model used to study the rewarding and aversive effects of drugs, was not observed
Potential delivery device
- Neurons are exposed within the olfactory area of the nares4, facilitating the transport of drug compounds directly into the brain
- For nose-to-brain delivery, the dose must be deposited in the olfactory region, and thus, a specialized delivery device is required
- A propellant activated device has been identified that will be studied to deliver encapsulated enkephalin

Support
- The National Institutes of Health (NIH) HEAL (Helping to End Addiction Long-term) Initiative is a government sponsored effort to speed scientific solutions to stem the national opioid public health crisis5
- Under the NIH HEAL Initiative, Virpax Pharmaceuticals entered a collaborative research and development agreement through the National Center for Advancing Translational Sciences (NCATS) of the NIH to study the pharmacology and safety of an intranasal enkephalin formulation for the treatment of pain
Conclusion
- An estimated 50 million (20%) of U.S. adults had chronic pain, with 20 million (8%) having high-impact chronic pain6—a debilitating medical condition that is complex and lacks effective treatments. The development of new therapies as viable alternatives to mu-opioids is urgently needed
- More than a million people have died from drug overdose – largely opioids – in the last two decades since the CDC began collecting data. Deaths from opioid overdose rose from 69,061 in 2020 to 80,926 in 2021, a rise of 17.2% in one year1. More than 106,000 people in the U.S. died from a drug-involved overdose in 2021, including illicit drugs and prescription opioids7
These data do not reflect a significantly greater number of non-fatal overdoses - Enkephalins are endogenous peptides reported to have a number of central and peripheral physiological effects. We tested the hypothesis that the nanoparticle encapsulation of enkephalin would lead to effective brain delivery and analgesia. The formulation studied above appears to protect enkephalin from rapid degradation on its nasally administered course through the olfactory neurons into the CNS
MET/L-ENK appeared effective in animal models of analgesia without obvious tolerance, and failed to show evidence of drug liking. Should studies advance in humans and translate to similar findings of safety and efficacy, this formulation has the potential to be a safe and effective analgesic of the future.
SUMMARY
Our data shows enhanced enkephalin transport to the brain using a novel Molecular Envelope Technology (MET), yielding analgesic effects in multiple preclinical models.
If data confirms analgesic benefit without respiratory depression or addiction, this enkephalin formulation may represent a potential broad-spectrum molecule to treat multiple types of acute and chronic pain and other CNS disorders.
Have a question?
Learn more about our work and inquire about unique opportunities with our delivery systems and formulations. Make a difference in the pain management industry.